The maker of Taytulla birth-control treatment issued a nationwide recall in the United States on Tuesday over concerns that misplaced capsules in pill packs could cause unintended pregnancies.
A physician reported that four placebo capsules were packaged in the wrong order where active capsules should have been, drugmaker Allergan said in a statement.
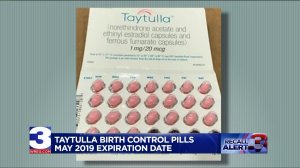
The physician sample pack should have 24 pink capsules with hormones followed by four maroon capsules without hormones, Allergan said. Instead, the faulty pack had four maroon placebos at the start of the treatment.
“As a result of this packaging error, oral contraceptive capsules that are taken out of sequence may place the user at risk for contraceptive failure and unintended pregnancy,” the statement said.
“The reversing of the order may not be apparent to either new users or previous users of the product, increasing the likelihood of taking the capsules out of order.”
Allergan said it is arranging for return of all sample pack products with the lot #5620706 Exp. May 2019. The drug maker urged patients to consult their physicians if they think they are affected by the recall.
Stroller recall
The U.S. Consumer Product Safety Commission also announced a recall involving a popular brand of strollers.
According to the agency, Jane Muum strollers don’t meet the proper federal safety standards. The company has found a child can slip through the opening between the stroller armrest and seat, posing a potential entrapment and strangulation hazard.
The strollers were sold at stores like Albee Baby and Toys R Us.
Call (844) 200-7971 for a free repair kit.



















